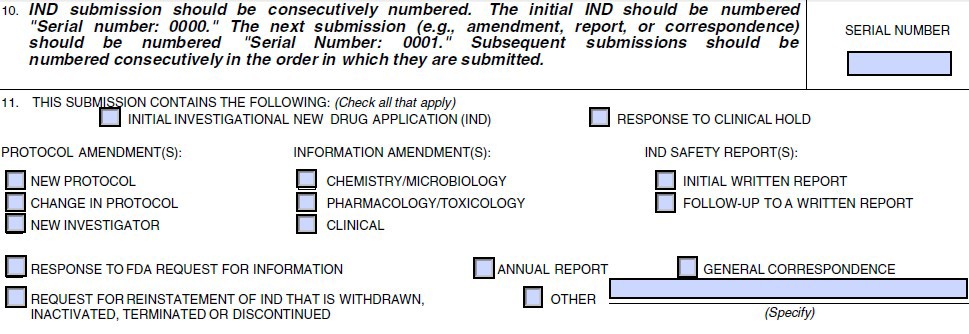
Form FDA 1572 Instructions General Information and Instructions This form instruction is to assist clinical investigators in com

Compliance4alllearning - Form FDA 1572 is one of the primary documents needed when carrying out a clinical trial. Also called the Statement of Investigator; Form FDA 1572, called just 1572 informally, is

The Investigator's Guide to Form FDA 1572: Getting the Statement of Investigator Right | CenterWatch